Clostridium difficile Glutamate Dehydrogenase (GDH) Antigen Rapid Test
Rapid detection of C. difficile Glutamate Dehydrogenase (GDH) antigen in stool samples for CDI screening.
Why Choose reOpenTest Gastrointestinal Health Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Fast Screening
Rapidly screen for C. difficile with a turnaround time of 10-15 minutes.
Initial Indicator
A positive result suggests the need for further confirmatory testing.
Versatile Use
Suitable for both clinical laboratories and point-of-care settings.
Product Overview
The C. difficile Glutamate Dehydrogenase (GDH) Antigen Rapid Test is a qualitative immunoassay for detecting C. difficile GDH antigen in stool. It aids in the initial screening for CDI. Rapid turnaround enables timely management and control.
Technical Specifications
| Detection Target | Clostridium difficile Glutamate Dehydrogenase (GDH) antigen |
|---|---|
| Sample Type | Stool |
| Methodology | Immunochromatography |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 18 months |
Procedure & Interpretation
Step-by-Step Procedure
- Add the stool sample to the provided buffer.
- Mix well, then add drops to the test cassette sample well.
- Read the result in 10-15 minutes.
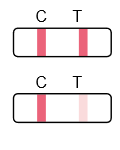
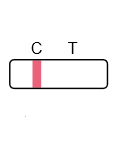
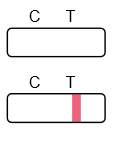
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.