Coronavirus (COVID-19)/Influenza A/B (Flu A/B)/Respiratory Syncytial Virus (RSV)/Adenovirus (Adeno)/Mycoplasma pneumoniae (M.P)/Streptococcus A (Strep A)/Chlamydia pneumoniae (C.P)/Human Metapneumovirus (HMPV)/Human Parainfluenza Virus 1/3/12 (HPIV 1/3/12) Antigen Combo Rapid Test
Simultaneous detection of 9 common respiratory pathogens from a single nasal swab.
Why Choose reOpenTest Infectious Disease Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Extensive Detection
Detects 9 common respiratory pathogens.
Rapid Results
Provides results in minutes, facilitating quick clinical decisions.
Easy to Use
User-friendly format minimizes training requirements.
Product Overview
This multi-analyte rapid diagnostic test simultaneously detects antigens from nine common respiratory pathogens: Coronavirus (COVID-19), Influenza A, Influenza B, Respiratory Syncytial Virus (RSV), Adenovirus, Mycoplasma pneumoniae, Streptococcus A, Chlamydia pneumoniae, and Human Parainfluenza Viruses 1, 3, and 12. This test is intended for professional use to aid in the rapid diagnosis of respiratory infections.
Technical Specifications
| Detection Target | COVID-19, Influenza A, Influenza B, RSV, Adenovirus, Mycoplasma pneumoniae, Streptococcus A, Chlamydia pneumoniae, HPIV 1/3/12 antigens |
|---|---|
| Sample Type | Nasal swab |
| Methodology | Immunochromatographic assay |
| Detection Range | Qualitative (Positive/Negative) |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect nasal swab specimen.
- Add the extraction buffer into the extraction tube.
- Insert the swab into the extraction tube and rotate the swab vigorously for 15 seconds.
- Add 3 drops of the processed specimen to the sample well of the test device.
- Read the results at 10-15 minutes.
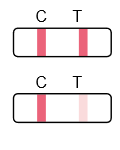
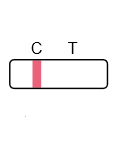
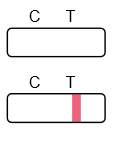
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.