Hepatitis C Virus (HCV) Antibody Rapid Test Device (Whole Blood/Serum/Plasma)
Rapid HCV antibody detection in whole blood, serum, or plasma.
Why Choose reOpenTest Infectious Disease Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Flexible Sample Types
Compatible with whole blood, serum, and plasma.
Quick Turnaround
Results available in 10-15 minutes.
Easy to Use
Simple lateral flow assay.
Product Overview
The Hepatitis C Virus (HCV) Antibody Rapid Test Device is a qualitative immunoassay for the detection of antibodies to HCV in human whole blood, serum, or plasma samples. This point-of-care test provides a rapid and convenient method for screening individuals for the presence of HCV antibodies, indicating past or current infection.
Technical Specifications
| Detection Target | HCV Antibodies |
|---|---|
| Sample Type | Whole Blood, Serum, Plasma |
| Methodology | Immunochromatography (Lateral Flow Assay) |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect a whole blood, serum, or plasma sample.
- Apply the sample to the test device.
- Add the provided buffer solution.
- Read the results after 10-15 minutes.
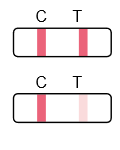
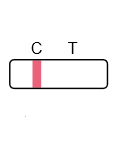
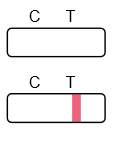
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.