Mycoplasma pneumoniae Antigen (MP Ag) Rapid Test
Quickly detect Mycoplasma pneumoniae antigen (MP Ag) in respiratory samples for timely diagnosis.
Why Choose reOpenTest Infectious Disease Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Fast Results
Provides results in minutes for timely clinical decisions.
Easy Sample Collection
Compatible with nasal and throat swab samples.
Point-of-Care Testing
Simple procedure suitable for clinics, offices, and emergency rooms.
Product Overview
The Mycoplasma pneumoniae Antigen (MP Ag) Rapid Test is a qualitative immunoassay for the rapid detection of Mycoplasma pneumoniae antigen in respiratory samples. This point-of-care test provides results within minutes, aiding in the timely diagnosis and management of Mycoplasma pneumoniae infections. Early detection is crucial for effective treatment and preventing complications. The test utilizes a simple procedure, minimizing the need for extensive laboratory equipment or specialized personnel. This test is designed for use in various healthcare settings, from clinics and physician offices to emergency rooms, providing efficient and accessible diagnostics for respiratory infections.
Technical Specifications
| Detection Target | Mycoplasma pneumoniae Antigen (MP Ag) |
|---|---|
| Sample Type | Nasal swab, throat swab |
| Methodology | Immunochromatography |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 18 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect nasal or throat swab sample.
- Add the swab to the provided buffer solution.
- Apply the processed sample to the test device.
- Wait 10-15 minutes and read the results.
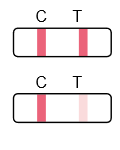
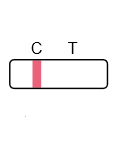
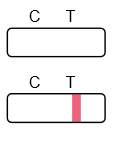
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.