Tick-Borne Encephalitis Virus (TBEV) Rapid Test
Rapid detection of TBEV IgM antibodies for early diagnosis of tick-borne encephalitis.
Why Choose reOpenTest Infectious Disease Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Early Diagnosis
Facilitates early diagnosis of TBEV infection, reducing severity and complications.
Rapid Results
Provides results in 10-15 minutes.
Point-of-Care
Suitable for point-of-care testing with minimal equipment needed.
Product Overview
The Tick-Borne Encephalitis Virus (TBEV) Rapid Test is a qualitative immunoassay designed for the rapid and sensitive detection of TBEV IgM antibodies in human serum or plasma samples. This point-of-care test aids in the early diagnosis of tick-borne encephalitis, a serious viral infection transmitted through the bite of infected ticks. The test provides results within minutes, allowing for timely initiation of appropriate treatment and management of patients suspected of having TBEV infection. Early diagnosis is crucial in reducing the severity and long-term complications associated with TBEV. The test employs a highly specific antibody-antigen interaction for accurate results and utilizes a user-friendly format to minimize the need for specialized equipment or expertise.
Technical Specifications
| Detection Target | TBEV IgM antibodies |
|---|---|
| Sample Type | Serum or Plasma |
| Methodology | Immunochromatographic assay |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect serum or plasma sample.
- Apply the sample to the test cassette.
- Add the buffer solution.
- Read the results after 10-15 minutes.
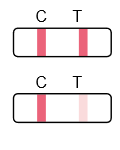
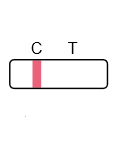
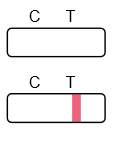
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.