Human Papillomavirus (HPV) Virus Stain
Visualize HPV in cervical, anal, and oropharyngeal specimens. Aids in identifying HPV, a key risk factor for cancers.
Why Choose reOpenTest Others Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Enhanced Visualization
Clearly visualizes koilocytes, characteristic cells of HPV infection.
Accurate Diagnosis
High-quality stain ensures consistent results for accurate diagnosis.
Early Detection
Facilitates early detection and intervention for potential cancer prevention.
Product Overview
The Human Papillomavirus (HPV) Virus Stain is a diagnostic tool used in cytology laboratories for detecting and visualizing HPV in cervical, anal, and oropharyngeal specimens. It helps identify HPV, a significant risk factor for various cancers, including cervical cancer. The stain enhances koilocyte visualization, enabling pathologists to assess infection severity and guide clinical management. Ensures clear, consistent results for accurate diagnosis and treatment decisions, contributing to early detection and cancer risk reduction.
Technical Specifications
| Detection Target | Human Papillomavirus (HPV) |
|---|---|
| Sample Type | Cervical, anal, and oropharyngeal smears (fixed and processed) |
| Methodology | Immunocytochemistry or Histochemical staining |
| Detection Range | Qualitative - Presence or absence of HPV-associated cytopathic changes |
| Reaction Time | Varies depending on staining protocol, approximately 30-60 minutes |
| Storage Conditions | 2-8u00b0C |
| Shelf Life | 12 months |
Procedure & Interpretation
Step-by-Step Procedure
- Fix and process tissue samples (cervical, anal, oropharyngeal).
- Apply the HPV Virus Stain to the prepared sample.
- Follow the recommended staining protocol, including incubation times and washing steps.
- Examine the stained sample under a microscope to identify HPV-associated cytopathic changes.
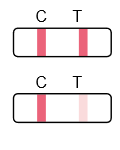
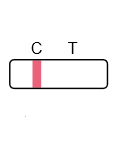
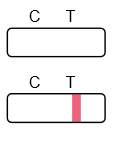
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Related Products
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.