Ethyl Glucuronide (ETG) One Step Test Device (Urine)
Convenient ETG urine test device for detecting recent alcohol use.
Why Choose reOpenTest Toxicology & Drug Monitoring Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
All-in-One Device
Self-contained device for easy urine collection and testing.
Accurate Results
Reliable detection of ETG with clear visual results.
Professional Use
Designed for healthcare professionals and trained personnel.
Product Overview
The Ethyl Glucuronide (ETG) One Step Test Device is a rapid, qualitative immunoassay for the detection of ethyl glucuronide (ETG) in urine. This device provides a convenient and reliable method for screening individuals for alcohol use, particularly useful in monitoring compliance with abstinence programs, assessing relapse risk, and assisting in forensic toxicology.
Technical Specifications
| Detection Target | Ethyl Glucuronide (ETG) |
|---|---|
| Sample Type | Urine |
| Methodology | Immunochromatography |
| Detection Range | Positive/Negative |
| Reaction Time | 5-10 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect urine sample directly into the device.
- Close the lid and wait for the reaction to occur.
- Read the results after 5-10 minutes.
Result Interpretation
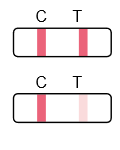
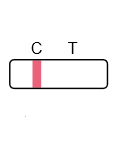
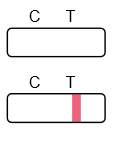
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Related Products
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.