Gamma-Hydroxybutyric Acid/Ketamine Combo Rapid Test (GHB/KET)
Simultaneous detection of GHB and Ketamine in urine.
Why Choose reOpenTest Toxicology & Drug Monitoring Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Dual Detection
Simultaneously detects GHB and Ketamine for comprehensive screening.
Rapid Results
Provides presumptive results within minutes for quick decision-making.
Professional Use
Intended for clinical and forensic settings, aiding informed decisions.
Product Overview
The Gamma-Hydroxybutyric Acid/Ketamine Combo Rapid Test (GHB/KET) is an immunochromatography based one step in vitro test. It is designed for qualitative determination of Gamma-Hydroxybutyric acid (GHB) and Ketamine in human urine specimens.
Technical Specifications
| Detection Target | GHB and Ketamine |
|---|---|
| Sample Type | Urine |
| Methodology | Immunochromatography |
| Detection Range | Qualitative (Positive/Negative) |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect urine sample in a clean, dry container.
- Dip the test strip into the urine sample for the specified time.
- Read the results within 10-15 minutes.
Result Interpretation
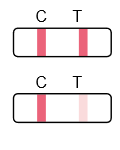
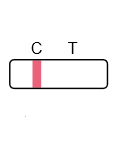
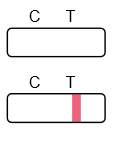
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.