Rapid Hydromorphone Test
Hydromorphone Rapid Test
Quick detection of Hydromorphone in urine samples.
CE Certified
ISO 13485
Why Choose reOpenTest Toxicology & Drug Monitoring Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Rapid Results
Provides results within minutes for timely decisions.
Professional Use
Designed for use by healthcare professionals.
Urine Sample
Easy to collect and test urine samples.
Product Overview
The Hydromorphone Rapid Test is a rapid screening test for the qualitative detection of Hydromorphone in urine.
Technical Specifications
| Detection Target | Hydromorphone |
|---|---|
| Sample Type | Urine |
| Methodology | Immunochromatography |
| Detection Range | Positive/Negative |
| Reaction Time | 10 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 18 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect a urine sample in a clean container.
- Dip the test strip into the urine sample.
- Read the results after 10 minutes.
Result Interpretation
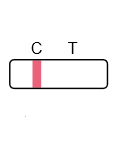
Positive:
A colored line appears in the control region (C) only.
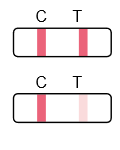
Negative:
Colored lines appear in both the control region (C) and the test region (T).
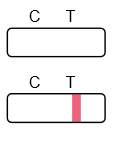
Invalid:
No line appears in the control region (C).
Frequently Asked Questions
The cutoff concentration is specified in the product insert.
This test is designed to be specific for Hydromorphone. Please refer to the product insert for cross-reactivity information.
A positive result is presumptive and should be confirmed with a more specific laboratory method. Other factors may influence the result.
Technical Documents
Download product inserts and brochures for detailed information.
No technical documents are available at this time. Please check back soon.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.