Synthetic Cannabis (K3) One Step Test Device (Urine)
Convenient device for detecting K3 synthetic cannabis in urine.
Why Choose reOpenTest Toxicology & Drug Monitoring Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Easy Sample Application
Integrated device simplifies urine sample application.
Fast Turnaround
Results available in just 5-10 minutes.
Reliable Screening
Accurate detection of K3 with high sensitivity.
Product Overview
The Synthetic Cannabis (K3) One Step Test Device (Urine) is a rapid, qualitative immunoassay for the detection of K3 in human urine. Offers a simple, efficient screening method. Employs a highly sensitive antibody for detecting low concentrations. Results in minutes, aiding timely decisions.
Technical Specifications
| Detection Target | K3 (Synthetic Cannabinoid) |
|---|---|
| Sample Type | Urine |
| Methodology | Immunochromatography |
| Detection Range | Qualitative (Positive/Negative) |
| Reaction Time | 5-10 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 24 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect urine sample in a clean container.
- Dispense 3 drops of urine into the sample well of the device.
- Read the results at 5 minutes.
Result Interpretation
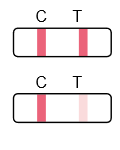
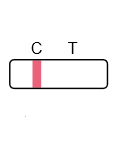
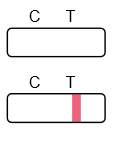
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.