Fetal Fibronectin (fFN) Rapid Test (Vaginal Secretion Swab)
Rapid detection of fetal fibronectin (fFN) in vaginal secretions.
Why Choose reOpenTest Women's Health & Reproduction Kits?
Our tests are engineered for accuracy, reliability, and ease of use, ensuring confidence in every result.
Timely Intervention
Allows for timely interventions to reduce the likelihood of preterm birth.
Point-of-Care
Provides quick results at the point-of-care, facilitating prompt decision-making.
Risk Assessment
Valuable tool for risk assessment of preterm delivery between 24 and 34 weeks.
Product Overview
The Fetal Fibronectin (fFN) Rapid Test detects fetal fibronectin in vaginal secretions between 24 and 34 weeks of gestation, indicating an increased risk of preterm delivery within two weeks. Provides healthcare professionals with a valuable risk assessment tool.
Technical Specifications
| Detection Target | Fetal Fibronectin (fFN) |
|---|---|
| Sample Type | Vaginal Secretion Swab |
| Methodology | Immunochromatographic assay |
| Detection Range | Positive/Negative |
| Reaction Time | 10-15 minutes |
| Storage Conditions | 2-30u00b0C |
| Shelf Life | 18 months |
Procedure & Interpretation
Step-by-Step Procedure
- Collect vaginal secretion sample using the provided swab.
- Apply sample to the test device.
- Wait 10-15 minutes.
- Read and interpret the results.
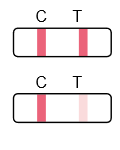
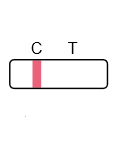
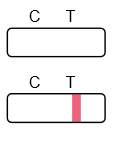
Result Interpretation
Frequently Asked Questions
Technical Documents
Download product inserts and brochures for detailed information.
Partner with reOpenTest
Provide your clients and patients with reliable diagnostic solutions.
Contact us today for bulk pricing and distribution opportunities.