Quality & Compliance
The verifiable foundation of trust in every product we deliver.
Quality is Not a Department, It's Our Operating System
For reOpenTest, quality is the bedrock of 'Verifiable Reliability,' designed into the core of our manufacturing architecture. We do not view compliance as a final inspection step, but as a commitment engineered at the source. Our rigorous Quality Management System (QMS) ensures that every component, every process, and every batch adheres to the most stringent global standards, eliminating the Black Box Risk for our partners and end-users.
Verifiable Adherence to Global Standards
ISO 13485:2016
Demonstrates our ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
CE Mark (IVDD/IVDR)
Certifies that our products meet the European Union's stringent safety, health, and environmental protection requirements for in-vitro diagnostic devices.
FDA Establishment Registration
Our manufacturing facilities are registered with the U.S. Food and Drug Administration (FDA) and are subject to their rigorous inspection and quality system regulations (QSR).
Verify RegistrationMDSAP
Participating in MDSAP allows a single regulatory audit of our QMS to satisfy the requirements of multiple global regulatory authorities.
Learn MoreEngineered for Compliance, Validated by the World
These global certifications are the irrefutable, external validation of the operational excellence driven by our NomoFlow™ platform. NomoFlow™ was designed from first principles to exceed these stringent benchmarks, utilizing AI-driven quality prediction and control.
This means rigorous compliance is a natural, predictable output of our process, ensuring 'Source Certainty' and simplifying market access for our global partners.
Explore the NomoFlow™ Platform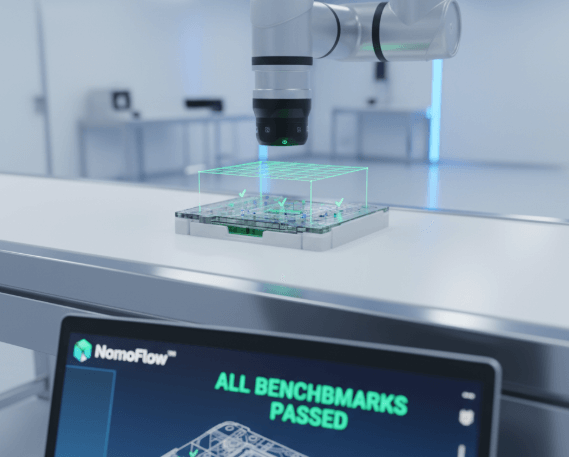
Partner with Uncompromising Quality
Our unwavering commitment to quality and global compliance provides our partners with a powerful advantage: confidence. Deliver products to your market that are backed by verifiable, world-class standards.
Explore Partnership Programs